Monomers and Polymers
What are monomers?
Think of monomers as small building blocks – tiny, similar molecules that can stick together to make bigger molecules, which we call polymers.What are polymers?
Polymers are like the large structures or creations made by connecting many of these small building blocks, the monomers. They're the big things formed when lots of those little things come together.

Monomers: Important Building Blocks to Know
Monomers are fundamental units, and examples include nucleic acids, amino acids, α&β glucose, fructose, fatty acids, and glycerol. Isomers are molecules with the same molecular formula but different molecular arrangements.
DNA and RNA Nucleotides
A nucleotide consists of three parts:
1. A nitrogenous base
2. A pentose sugar
3. One or more phosphate groups
Nucleotides have two types of pentose sugar: deoxyribose (found in DNA) and ribose (found in RNA). Deoxyribose is similar to ribose but has an H instead of an OH at the 2’ positions.
Diagram of Nucleotides (DNA and RNA)

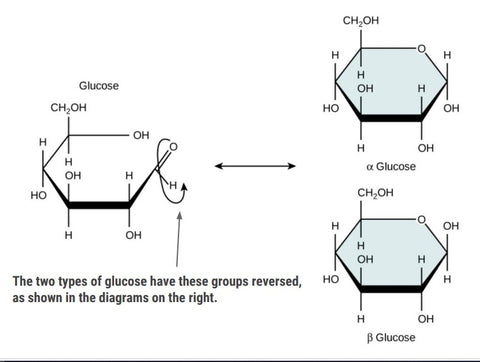
Glucose: Understanding the Basics
Glucose is a hexose sugar, specifically a monosaccharide, with six carbon atoms in each molecule.
There are two types of glucose: alpha (α) and beta (β). They are isomers, indicating that they share the same molecular formula, but their atomic connections differ.
For your exam, it's important to be familiar with the structure of both alpha and beta glucose. Luckily, distinguishing between them is straightforward, as illustrated in the diagram below, where only one difference separates the two.
Other Essential Monomers: Fructose and Amino Acids
In preparation for your exams, it's crucial to understand two key monomers: fructose and amino acids. Let's explore them briefly.
-
Fructose:
- Fructose is one of the monosaccharides and serves as a vital building block for larger molecules.
- It is a sweet-tasting sugar commonly found in fruits and honey.
- In the context of polymers, fructose can contribute to the formation of more complex carbohydrates.
-
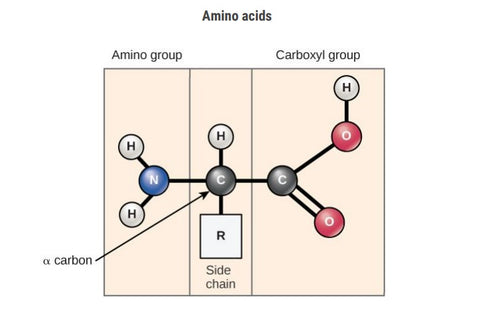
Amino Acids:
- Amino acids are fundamental units that make up proteins, a crucial component in living organisms.
- There are various amino acids, each with a unique structure and role in protein synthesis.
- Understanding the diversity of amino acids is essential for grasping the complexities of protein formation and function.

Essential Polymers: Carbohydrates, Proteins, and Lipids
To excel in your understanding of biology, it's vital to grasp the key polymers: Carbohydrates, Proteins, and Lipids. Let's delve into their basic characteristics.
1. **Carbohydrates:**
- Carbohydrates are crucial energy sources and structural components in living organisms.
- Monomers, such as glucose, are connected by glycosidic bonds to form larger carbohydrate molecules.
- Understanding carbohydrate structures and functions is essential for comprehending energy storage and cellular processes.
2. **Proteins:**
- Proteins are essential for various biological functions, including enzymes, structural support, and transport.
- Amino acids serve as the monomers in protein structures, linked together by peptide bonds.
- Exploring the diversity of proteins and their roles is fundamental to a comprehensive understanding of cellular activities.
3. **Lipids:**
- Lipids play key roles in energy storage, insulation, and forming cell membranes.
- Monomers, such as fatty acids and glycerol, are connected by ester bonds to create lipid molecules.
- Recognizing the significance of lipids in cellular structure and function is crucial for a holistic grasp of biological processes.


**Peptide Bond Formation: A Dehydration Synthesis Reaction**
Understanding how peptide bonds form is fundamental to comprehending protein synthesis. Here's a simplified breakdown:
- **Dehydration Synthesis Reaction:**
- Peptide bond formation is a type of dehydration synthesis, where two molecules combine with the removal of a water molecule.
- This process is crucial in building proteins, the workhorses of various biological functions.
- **Reaction Details:**
- The carboxyl group (-COOH) of one amino acid connects to the amino group (-NH2) of the incoming amino acid.
- This linkage results in the formation of a peptide bond between the two amino acids.
- Importantly, a water molecule is released as a byproduct during this coupling process.
Understanding the mechanics of peptide bond formation provides insights into the intricate molecular processes that govern the construction of proteins in living organisms
Triacylglycerol Formation: Ester Bond Synthesis
Understanding how triacylglycerol, a vital component in lipid storage, is formed involves the creation of ester bonds.
- Ester Bond Synthesis:
- Triacylglycerol formation is a result of an ester bond synthesis, a specific type of dehydration reaction.
- This process involves joining three fatty acids to a glycerol backbone.
- Reaction Details:
- Three fatty acids combine with a glycerol molecule, and the ester bonds form between the hydroxyl groups of glycerol and the carboxyl groups of the fatty acids.
- Crucially, this synthesis results in the release of three water molecules as byproducts.
Understanding the ester bond synthesis in triacylglycerol formation provides insights into how living organisms store energy in the form of lipids.

Test Yourself
**Multiple Choice Questions:**
**1. What are monomers in the context of polymers?**
- A. Large structures
- B. Complex carbohydrates
- C. Small, similar molecules
- D. Amino acids
**2. What is the primary function of polymers?**
- A. Formation of monomers
- B. Energy storage
- C. Structural support
- D. Dehydration synthesis
**3. Which of the following is an example of a monomer?**
- A. Glucose
- B. Carbohydrates
- C. Proteins
- D. Lipids
**4. What are isomers?**
- A. Different polymers
- B. Molecules with the same formula but different arrangement
- C. Monomers linked by glycosidic bonds
- D. Nitrogenous bases in nucleotides
**5. What is the unique feature of deoxyribose in DNA nucleotides?**
- A. Sweet taste
- B. Presence of OH at the 2’ positions
- C. Connection to glycerol backbone
- D. Isomer of ribose
**6. How many types of glucose are there, and what term describes their relationship?**
- A. One type; isomers
- B. Two types; isomers
- C. Three types; polymers
- D. Four types; monomers
**7. What is the function of fructose in the context of polymers?**
- A. Energy storage
- B. Structural support
- C. Formation of carbohydrates
- D. Sweet-tasting sugar
**8. Which bond connects amino acids in protein structures?**
- A. Glycosidic bond
- B. Ester bond
- C. Peptide bond
- D. Hydrogen bond
**9. What is the key role of lipids in living organisms?**
- A. Structural support
- B. Energy storage and insulation
- C. Formation of carbohydrates
- D. Peptide bond synthesis
**10. How is water involved in the formation of peptide bonds?**
- A. Water is consumed
- B. Water is a byproduct
- C. Water is a monomer
- D. Water breaks peptide bonds

